PHASE SEPARATION 101
About the project
Recent research has shown that cells are organized not only through membrane-bound organelles, but also through liquid droplets assembled by liquid–liquid phase separation (LLPS). This new paradigm, challenging the 20th century textbook view of cellular compartmentalization, is attracting growing attention as these biomolecular condensates, or biocondensates, seem to play important roles in cells' physiopathology. These concepts are now making its way into undergraduate classrooms.
This visual resource aims at helping students understand the basics of this emerging, intricate and dynamic concept. We would like to stress the fact that they are not molecular simulations; in particular, time scales have been slowed down for didactic purposes.
This project was designed and completed by Margot Riggi, under the supervision of Janet Iwasa, with funding provided by the European Molecular Biology Organization (EMBO). We thank all the researchers and educators who helped us with their valuable feedback along the way: Abby Dernburg, Andrew Folkmann, Jessica Fry, Amy Gladfelter, Alex Holehouse, Keren Lasker, Hyun Kate Lee, Rob Levenson, Beverly Naigles, Andrea Putnam, Christine Roden, Ofer Rog, Géraldine Seydoux, Elizabeth Villa, Stephanie Walker.
All animations can be downloaded on Vimeo.
Complementary resources
The following resources were designed and implemented by Margot Riggi, under the supervision of Janet Iwasa and in collaboration with Ofer Rog (University of Utah). This work was funded through NSF grant 2219605.
Contents
Chapter 1: Liquid-liquid Phase Separation is a form of phase transition
Chapter 2: Some important physico-chemical principles underlying Liquid-Liquid Phase Separation
Chapter 3: General characteristics of phase-separating proteins
Chapter 4: Cellular biomolecular condensates are highly complex systems
Chapter 5: Experimental techniques to study Liquid-Liquid Phase Separation
Chapter 6: Functional importance of Liquid-Liquid Phase Separation
Introduction: Cellular compartmentalization
Cells need to coordinate thousands of complex biochemical reactions at the same time in order to function properly. In some cases, high concentrations of specific molecules must localize at the right place at the right time. One solution to this fundamental problem is to compartmentalize cellular contents.
Traditionally, intracellular organization has been associated with compartments that are surrounded by lipid membranes, such as the nucleus, which contains most of a cell’s genetic material; mitochondria, responsible for energy production; and the endoplasmic reticulum, crucial for protein production. These membranes are impermeable to most biological molecules, physically separating the interior and exterior of classical organelles. Organelle compositions are thus regulated through specialized membrane transport machineries.
However, the field of cell biology is in the midst of a revolution as research has started to uncover the mechanisms through which the content of cells can also self-organize into membrane-less compartments of specific composition, termed “biomolecular condensates“ or “biocondensates”.
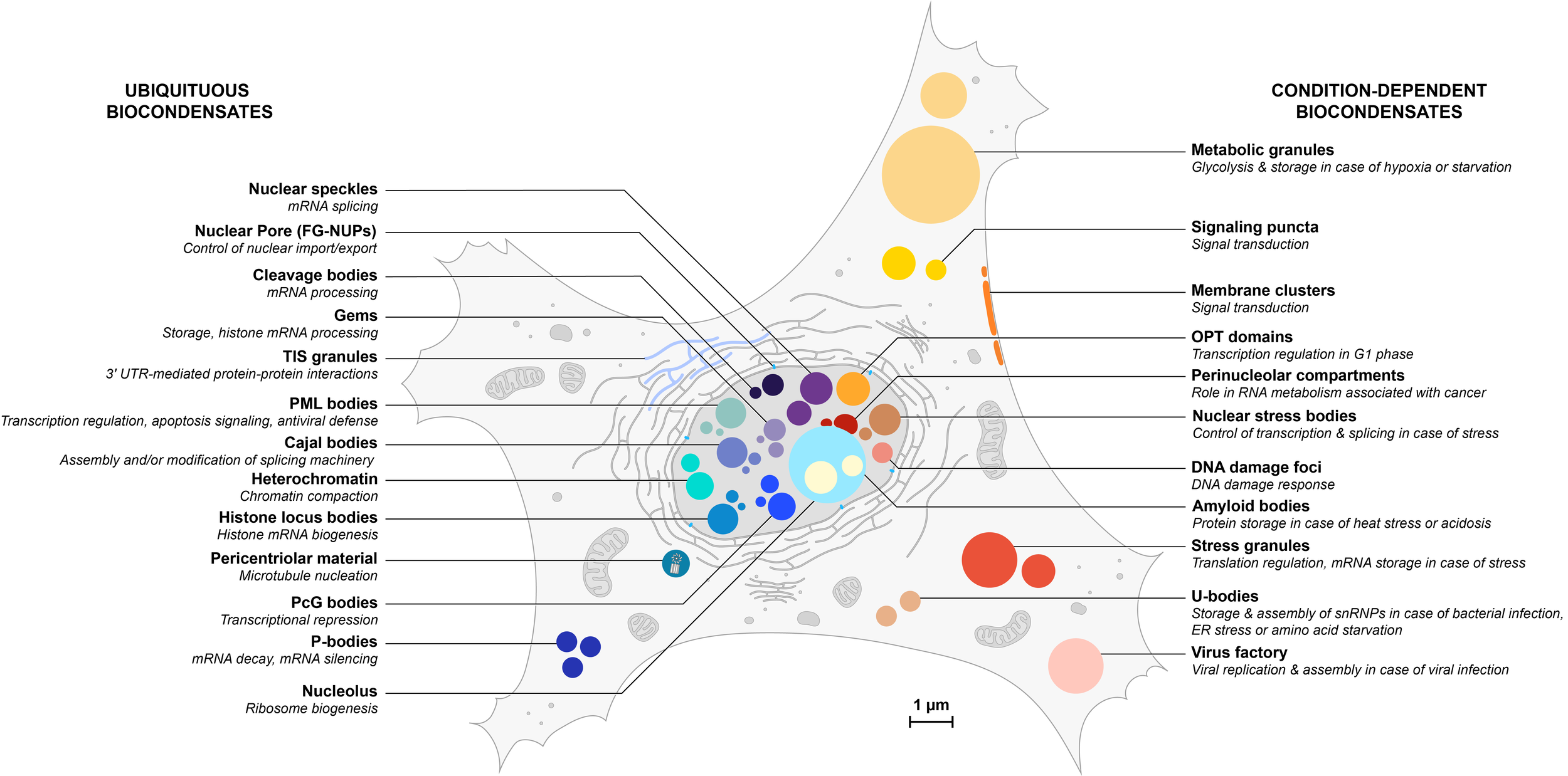
Overview of an increasing number of biocondensates in eukaryotic cells
Ubiquitous condensates are listed on the left and depicted in blue hues; condition-dependent condensates are listed on the right and depicted in orange hues. Some condensates (not represented here) are also cell-specific. All structures are represented to scale but their number has not been taken in consideration in this illustration.
Dynamic comparison between generic membrane-bound organelle (left) and biocondensate (right)
Chapter 1: Liquid-liquid Phase Separation is a form of phase transition
Physical states of matter
Solids, liquids, and gases are the most common states of matter.
Solid matter is composed of tightly packed molecules that have close, tight interactions with one another.
In a liquid, molecules can easily move, rearrange, and change their neighborhood quickly. As a consequence, a solid will retain its shape, while a liquid will take the shape of its container.
Gaseous matter is composed of particles that interact with one another even more weakly. As a result, the volume and shape of a gas can change dramatically depending on the conditions.
In each of these three states, the molecules themselves are chemically the same, but their organization is completely different, with dramatic consequences visible at the macroscopic level.
2. Phase transitions
Phase transition is the process by which a substance changes from one physical state to another. Commonly observed examples are phase transitions of water upon temperature change. For example, the freezing of water into ice (liquid to solid) or the heating of water to generate water vapor (liquid to gas).
The physical states of a substance under different conditions of temperature and pressure can be graphically summarized in a phase diagram.
3. Liquid-Liquid Phase Separation (LLPS)
Liquid–Liquid Phase Separation (LLPS) is a special form of phase transition by which one homogeneous solution spontaneously separates into two distinct immiscible liquids, or “phases”: a dense phase, and a dilute phase. It requires that at least one of the liquid components has a tendency to interact with itself more than with the other components, basically becoming a better solvent for itself than the buffer it is dissolved in. As a result, this component also becomes more concentrated in the dense phase.
In a similar manner as for phase transitions of water, LLPS can also be explained using a phase diagram.
These properties are not restricted to inorganic molecules. Biological macromolecules, such as proteins or nucleic acids, can also exist in different physical states and undergo similar phase transitions, which have emerged as a novel form of cellular organization.
Chapter 2: Important physico-chemical principles underlying LLPS
1. Entropy, interaction energy & Gibbs free energy
The second law of thermodynamics states that there is a natural tendency of any isolated system to evolve in the direction of increasing disorder. Why does phase separation, a process that, on the contrary, creates order, even happen?
Even though it is not highlighted in this animation, it is important to note that the solvent can also contribute to the interactions driving Liquid-Liquid Phase Separation.
2. Surface tension
Have you ever seen small insects walking on water? They can stay on the surface because of a phenomenon called surface tension, in which the surface of a liquid acts as a thin elastic sheet. The same phenomenon causes liquid droplets to adopt their characteristic spherical shape, and can be explained if we observe what happens at the molecular level.
3. Binding affinity
Phase separation of biopolymers is often driven by many weak interactions. How exactly do we define the strength of a molecular interaction?
Please note that the following animation is meant to define biomolecular binding in general rather than in the specific context of Liquid-Liquid Phase Separation.
Binding affinity is influenced by non-covalent intermolecular interactions, such as hydrogen bonds, electrostatic interactions, hydrophobic, and Van der Waals forces between the two molecules, and may be affected by the presence of other molecules.
Chapter 3: General characteristics of phase-separating proteins
Intracellular phase separation generally involves multiple weak interactions between multivalent macromolecules. These interactions need to be strong enough to bring molecules together, but not too strong to freeze their dynamics within the condensate.
Multivalency is the availability of several binding sites on the same molecule. It is a key molecular feature underlying the assembly of biocondensates.
In proteins, multivalent features can be manifested structurally in two main ways: modular proteins, and intrinsically disordered proteins. Importantly, folded domains and disordered regions can be found within the same protein, and both synergistically cooperate to drive phase separation.
RNAs can also contains both structured and unstructured regions.
Modular proteins
Intrinsically Disordered Proteins
Liquid-liquid Phase Separation of both disordered and folded macromolecules can be understood through the same “stickers-and-spacers” model, which reduces these macromolecules to two types of components, “stickers” and “spacers”.
Importantly, which individual amino acids are “sticky” in IDRs is not just an intrinsic property but also very much depends on the context. The distinction between stickers and spacers is therefore less sharp in this case.
Chapter 4: Cellular biomolecular condensates are highly complex systems
In vitro experiments enable scientists to study simplified systems, but biocondensates are much more complex in vivo.
We would also like to stress that in many cases the dilute phase (surrounding cytoplasm or nucleoplasm) is much larger than the biocondensate. As a result, molecules that enrich and become more concentrated in the biocondensates may be more abundant (in terms of number of molecules) in the dilute phase.
Beyond proteins and RNAs, some nuclear biocondensates can also contain DNA, and extracellular condensates, such as biofilms, can have complex sugars.
Chapter 5: Experimental techniques to study Liquid-Liquid Phase Separation
FRAP & molecular diffusion
Fluorescence Recovery After Photobleaching (FRAP) is a method for determining the diffusion kinetics of fluorescently-labeled molecules.
A few points to pay attention to:
- Different components of a droplet can have very different mobilities. In particular, RNAs are generally much less mobile than proteins.
- The recovery rate of a full-FRAP not only reports on the exchange rate between the dilute and the dense phases, but also depends on other parameters such as the size of the photobleached droplet, mobility within a droplet and the size of bleached areas.
2. Studying surface tension
Remember that surface tension is defined as the collective intermolecular forces that minimize surface area in order to lower the interface energy between two liquids.
2.1) Combining measures of the viscosity & the inverse capillary velocity of the biocondensate
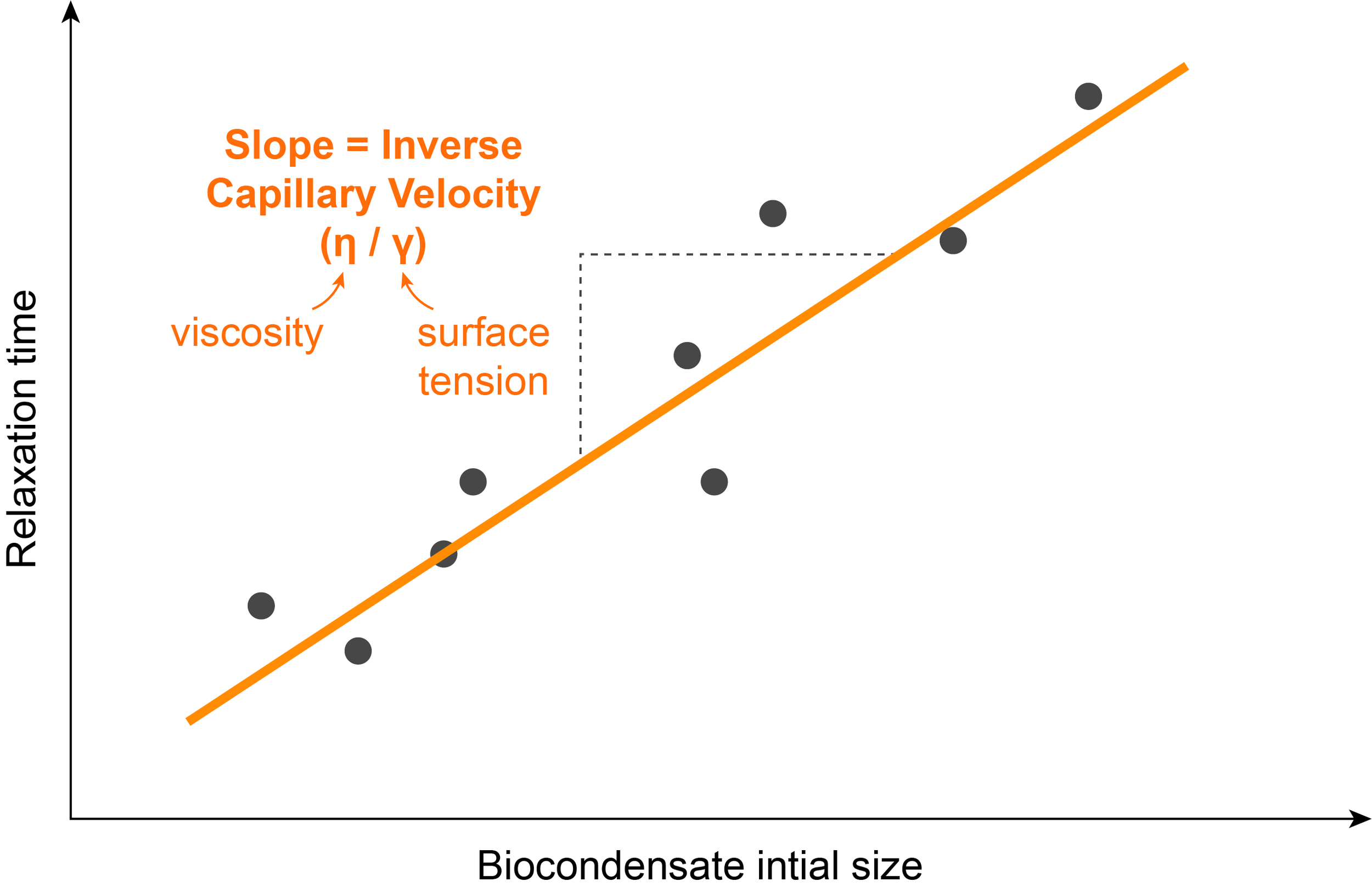
One assay used to determine the surface tension of a biocondensate in vitro is through the measure of a parameter called inverse capillary velocity, which is the ratio of the viscosity to surface tension. Combining measurements of inverse capillary velocity with other experiments in which viscosity can be directly calculated, allows for the calculation of the surface tension of droplets.
The inverse capillary velocity can be estimated by studying the kinetics of droplet fusion. It is possible to film two droplets fusing using fluorescence and measure the time they take to completely fuse into one droplet.
Repeating this experiment with droplets of different sizes shows a linear relationship between the fusion time and the initial radius of the droplets. The inverse capillary velocity (η/ϒ), corresponds to the slope of this line.
The viscosity of a fluid is a measure of its resistance to deformation. For liquids, it corresponds to the informal concept of "thickness": for example, honey has a higher viscosity than water.
Microrheology is a technique used to determine the viscosity of a medium by embedding uniform beads inside and tracking their position over time. At a given time, the mean squared displacement (MSD) is defined as the average of the distance between each bead and the reference position. It can be seen as the extent of their random motion.
This measure can be used to calculate the diffusion coefficient D of the droplet, using the following equation:
MSD = 2Dt
and then its viscosity η using the Stokes-Einstein equation:
D = kB T / 6𝜋 η Rh
where kB is the Boltzmann constant, T is the temperature (in Kelvin), η is the viscosity, and Rh is the hydrodynamic radius of the diffusing species (which corresponds to the size of a spherical particle that diffuses at the same rate than the molecule of interest).
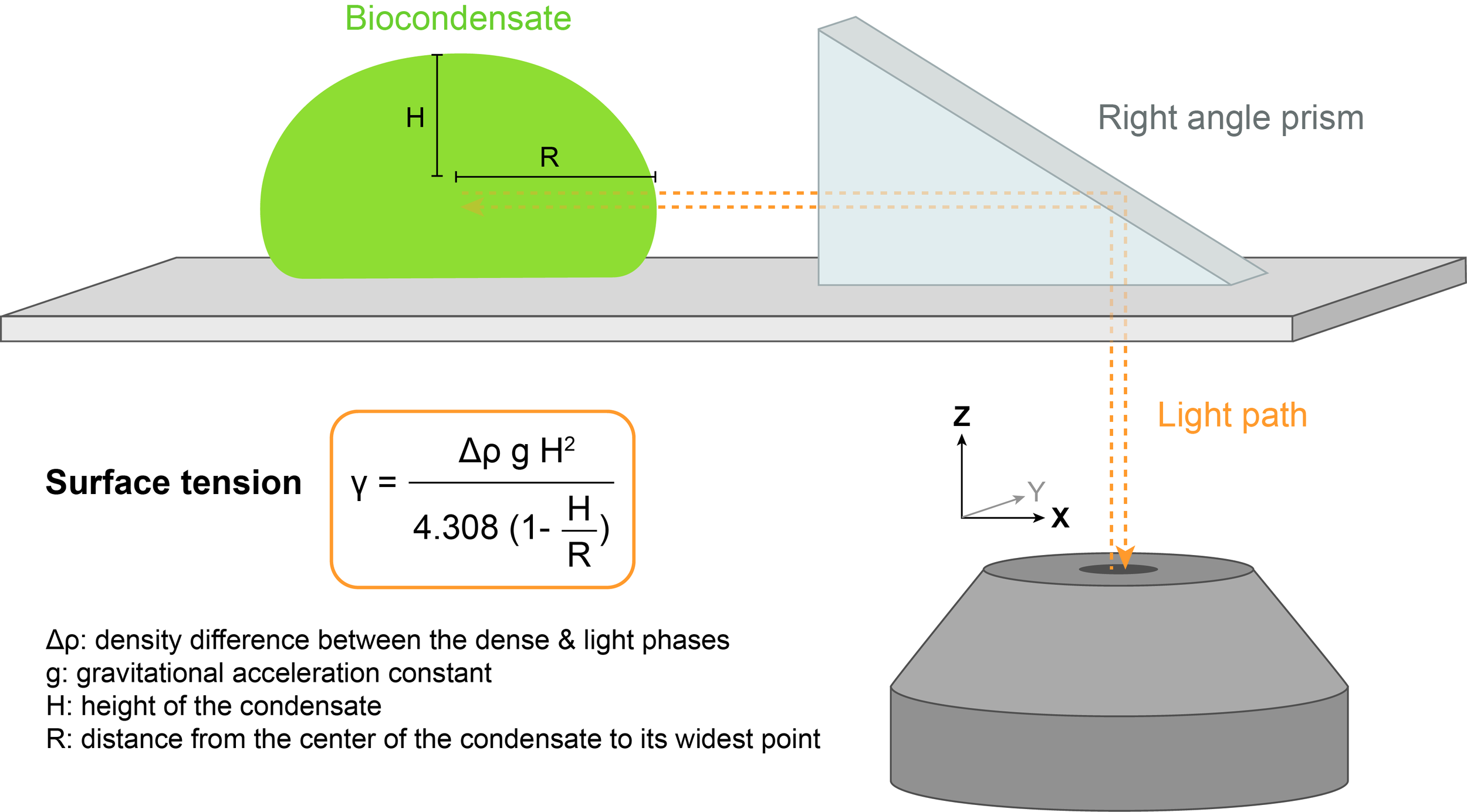
2.2) Right angle prism imaging
The shape of the biocondensate resting on a surface is dictated by a balance between surface tension, which promotes rounder droplets, and gravitational forces, which tend to flatten droplets. Surface tension 𝛾 can be determined by measuring the profile of the droplet in the XZ dimension.
A method called right angle prism imaging can be used to avoid imaging artifacts along the optical axis Z.
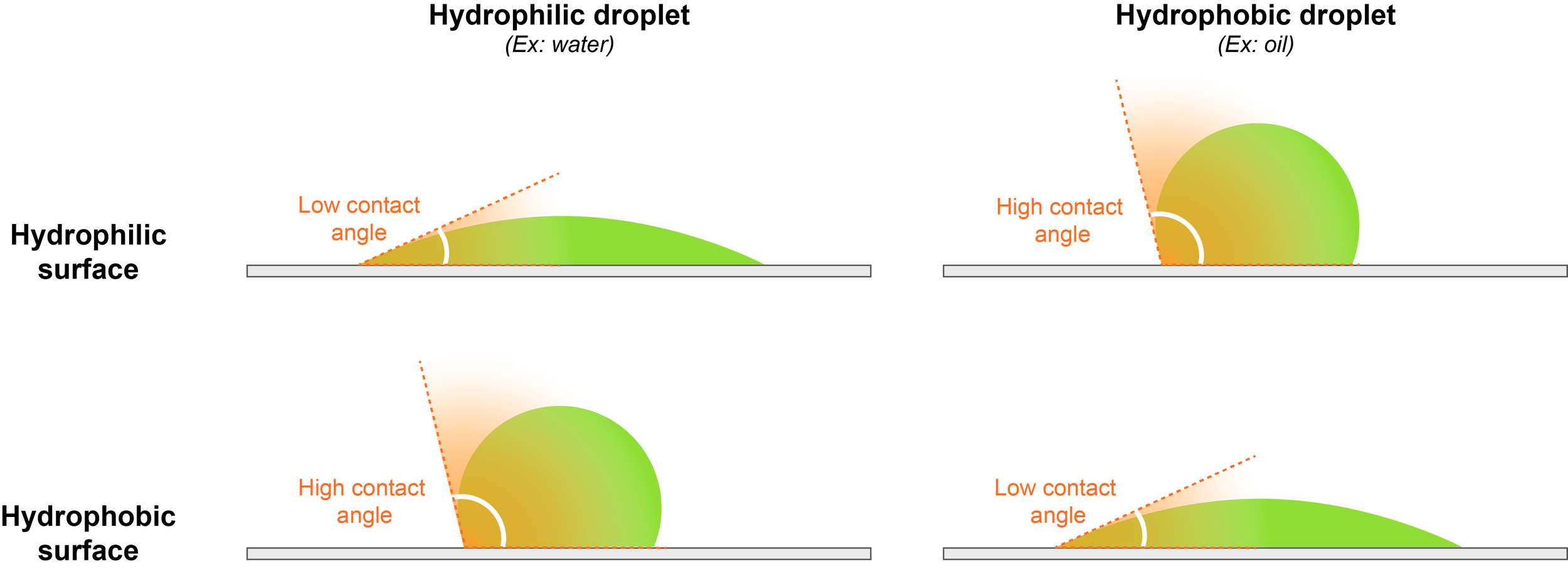
2.3) Assessment of the wetting behavior
Insights into the physicochemical properties of phase separated bodies can be also obtained by monitoring their interactions with hydrophobic and hydrophilic surfaces, in particular their wetting behavior. This can be done by measuring the angle between the coverslip and the droplet surface, also called contact angle.
For example, water droplets readily spread over a hydrophilic surface, thus showing a low contact angle, because the interaction between the droplet and the surface is energetically favorable. On the contrary, a water droplet will round up and avoid contact with a hydrophobic surface, displaying a high contact angle. This indicates energetically disfavored interactions between chemically incompatible hydrophilic droplets and the hydrophobic surface.
Chapter 6: Functional importance of Liquid-Liquid Phase Separation
Biocondensates are found throughout eukaryotic cells, where they are involved in a broad range of functions that we are only starting to understand. In many cases, these functions are still only hypotheses that remain to be fully tested in vivo. Because biocondensate components are also present in the dilute phase, mutants that eliminate these molecules are not sufficient to infer the function of the condensates, which makes this task very difficult.
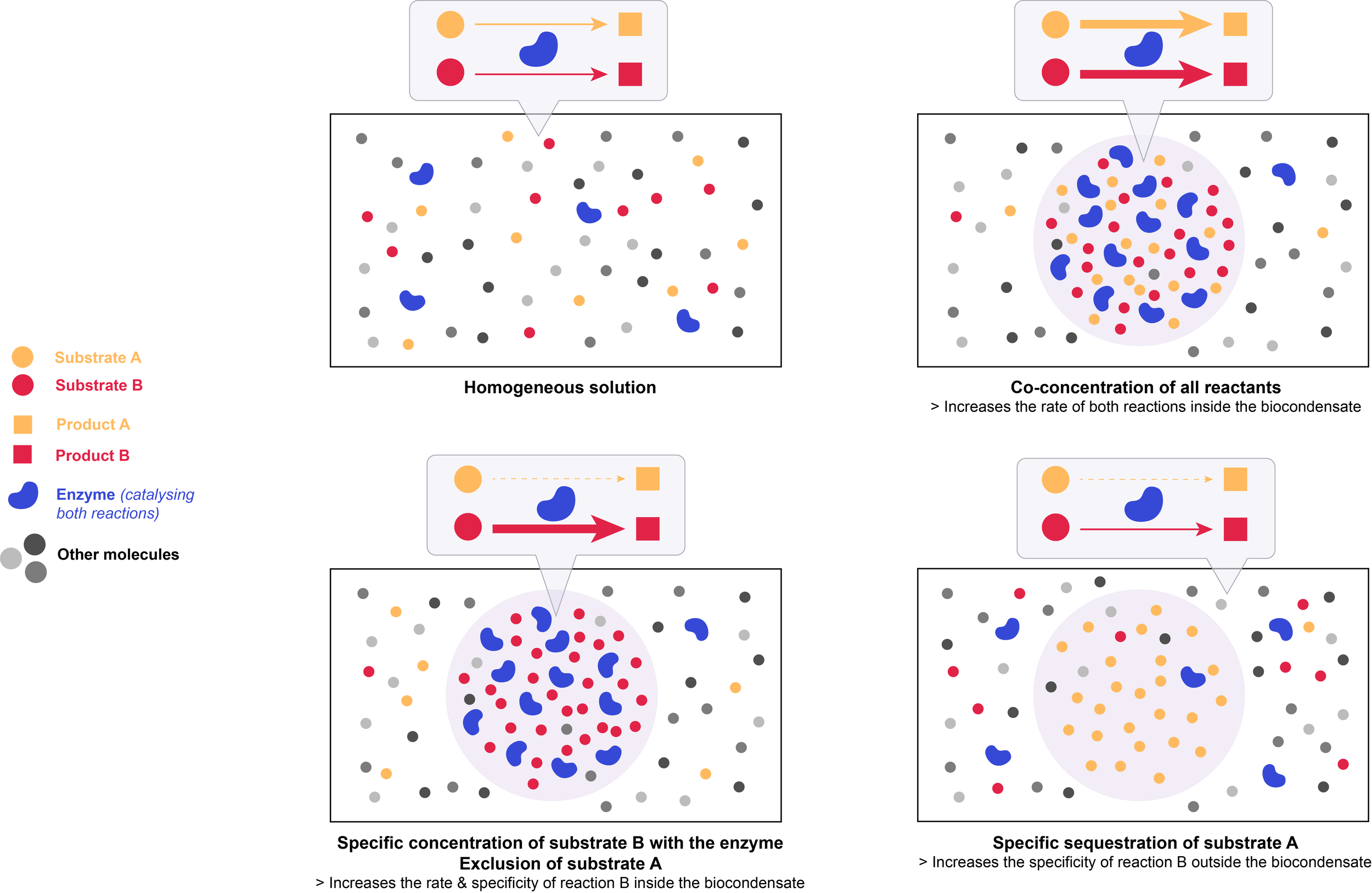
One of the key characteristics of biocondensates, important for their functions at all scales, is their ability to concentrate or exclude specific sets of molecules at specific locations in a quick and reversible manner.
1. At the molecular scale
The liquid-like nature of biocondensates allows for rapid motion of molecules, which promotes random collisions between reactants, and a dynamic exchange of reactants and products. Controlling local concentrations of specific molecules within biocondensates can influence both the kinetics and the specificity of biochemical reactions.
The law of mass action is the principle that the chemical reaction rate is proportional to the concentration of the reactants.
Co-concentrating all reactants thus increases the reaction kinetics inside the biocondensate.
Importantly, biocondensates have the ability to specifically concentrate certain molecules over others. Concentrating a molecule with only a subset of its potential partners, while excluding others, imparts specificity to biochemical processes: it promotes certain reactions and impedes others, thus reducing potential off-target effects. Excluding inhibitors (based on their charge or size for example) from the condensate will further promote the rate of a particular reaction within the biocondensate. This effect is especially important when reactants are present at concentrations well below their Km value, and will only be marginal if they are already saturated in the initial homogeneous solution.
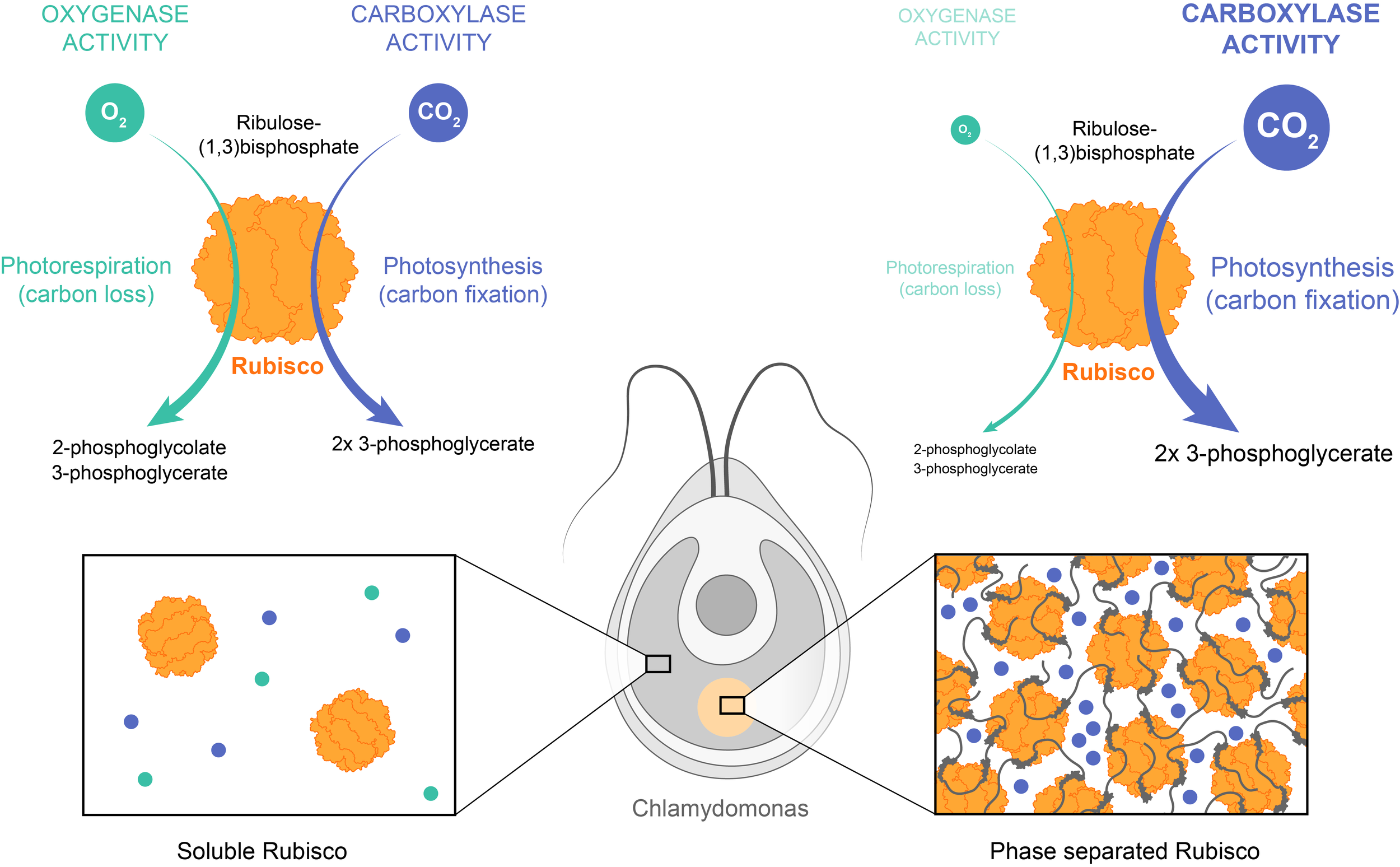
Example: Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is the major enzyme assimilating CO2 into the biosphere. However, it is a very slow enzyme and its carboxylase activity is compromised by an opposing oxygenase activity. To counter this, various photosynthetic organisms, including the unicellular algae Chlamydomonas, have independently evolved distinct multivalent ligands that interact with the octameric Rubisco to induce its phase separation. The resulting biocondensates concentrate Rubisco and carbon dioxide, but not O2. Phase separation thus enhances both the reaction rate, via enzyme and substrate co-concentration, and the reaction specificity because of preferential concentration of CO2 over the competing oxygen substrate.
On the contrary, sequestering molecules inside the condensate can also inhibit their activity outside the structure, decreasing the kinetics of reactions in the bulk phase.
Example: Paraspeckles are biocondensates found in the nucleus of mammalian cells. Their functions are not fully understood, but it is believed that they could be involved in transcription regulation, splicing factor sequestration, and nuclear retention of RNAs. The primary scaffold for paraspeckles is the long non-coding RNA, NEAT1, which recruits the transcription regulator protein SFPQ. Proteasome inhibition or viral infection upregulates NEAT1 transcription and enlarges paraspeckles, thereby depleting SFPQ by up to 50% from the nucleoplasm. This depletion then leads to repression of SFPQ target transcripts.
Condensates can also affect reactions by controlling the structural organization or dynamics of molecules. For example, increased viscosity may slow down the diffusion of molecules, thus decreasing the reaction kinetics. Scaffolding can tether reactants in close proximity, and consequently promote the reaction through decreased Km value.
2. At the (sub)cellular scale
2.1) Organization of the intracellular environment
Condensates form discrete structures that can localize molecules to specific locations within the cellular volume, thus controlling where reactions occur. This can organize and facilitate signaling at the cellular scale by co-localizing actors of the same pathway.
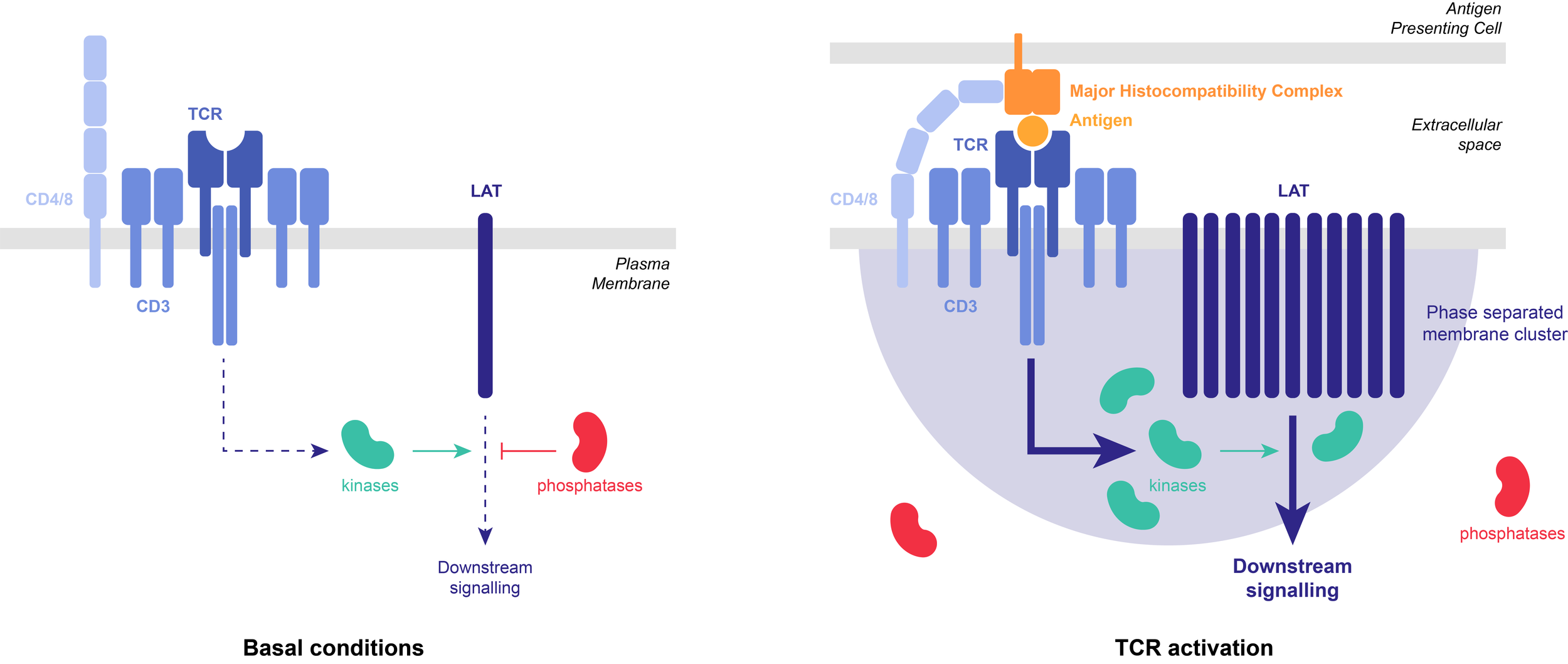
Example: T-cell receptors (TCR) can organize into membrane-associated 2D phase-separated clusters. TCR activation causes phosphorylation of the scaffold protein, LAT, which undergoes LLPS with a group of adaptor proteins. LAT phosphorylation is antagonized by the phosphatase CD45. Reconstituted in vitro LAT clusters partially exclude CD45, thereby stabilizing the clusters against dephosphorylation-triggered dissolution. Such positive feedback in LAT phosphorylation might serve to prolong TCR signaling and increase sensitivity to antigen stimulation.
The physical properties of condensates also allow them to alter the shape of subcellular structures and generate forces.
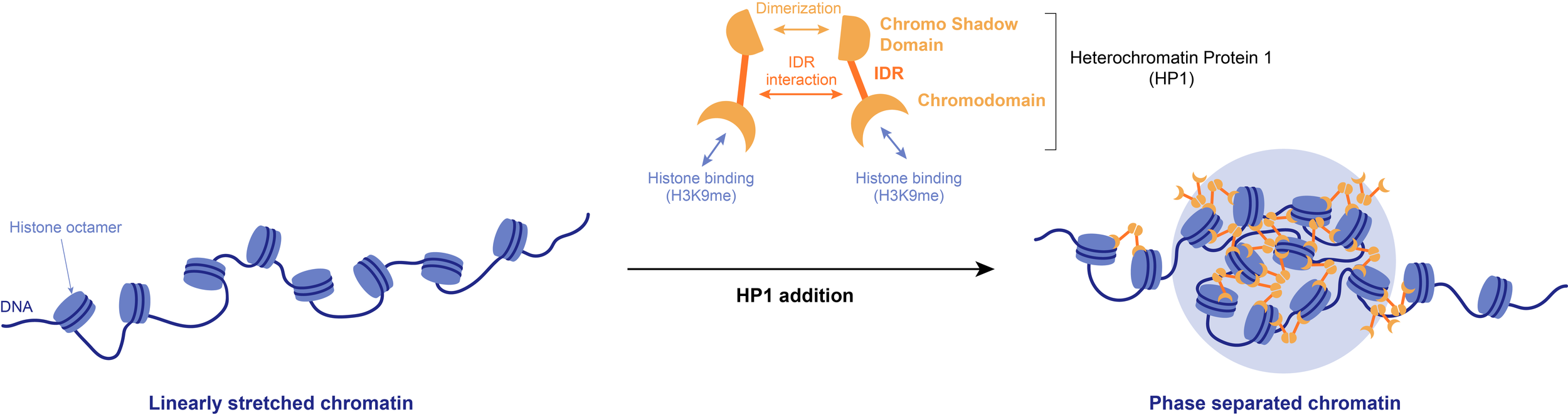
Example: Phase separation of the heterochromatin-promoting protein HP1 leads to the compaction of linearly stretched DNA. The resulting biocondensates may be necessary to sterically exclude certain factors, such as RNA polymerases, from heterochromatin.
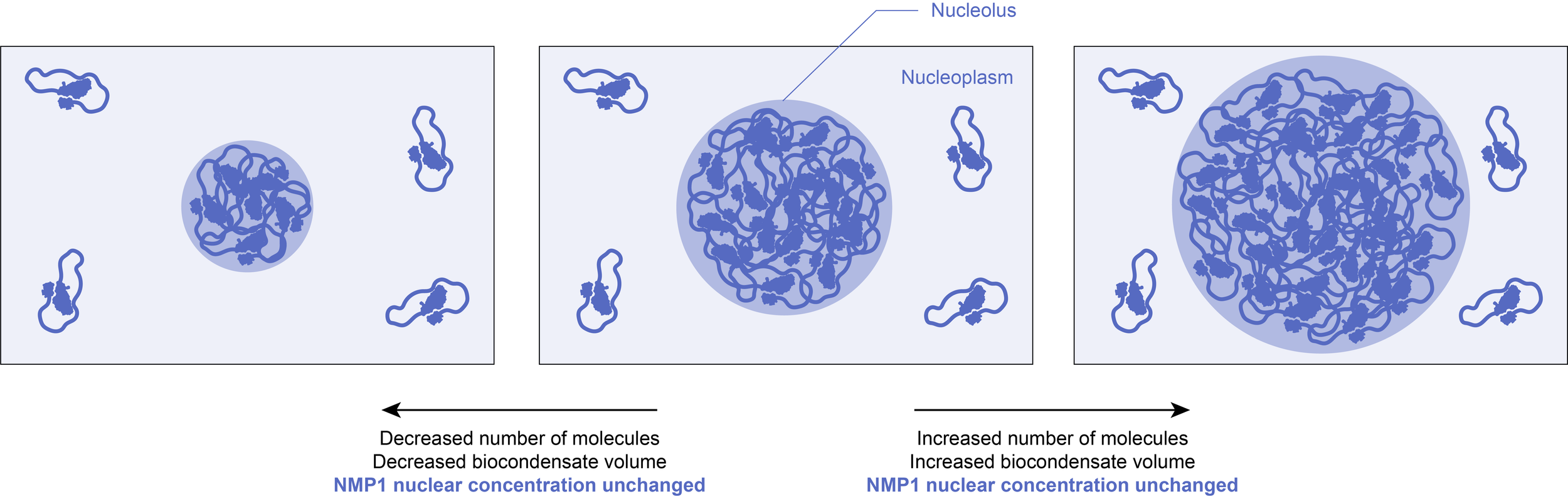
2.2) Buffering cellular noise
Once formed, the concentrations within the biocondensate and in the surrounding solution are fixed at the solubility threshold value. The volume of the condensed phase will thus grow if more molecules are added to the system, or diminish if the total number of molecules decreases. As a result, this could buffer against biological fluctuations (for example in gene expression), making certain pathways more robust to noise.
Example: It has been observed that phase separation of the endogenously expressed nucleolar component NPM1 in cells reduces fluctuations of NPM1 concentration in the nucleoplasm.
2.3) Maintaining homeostasis
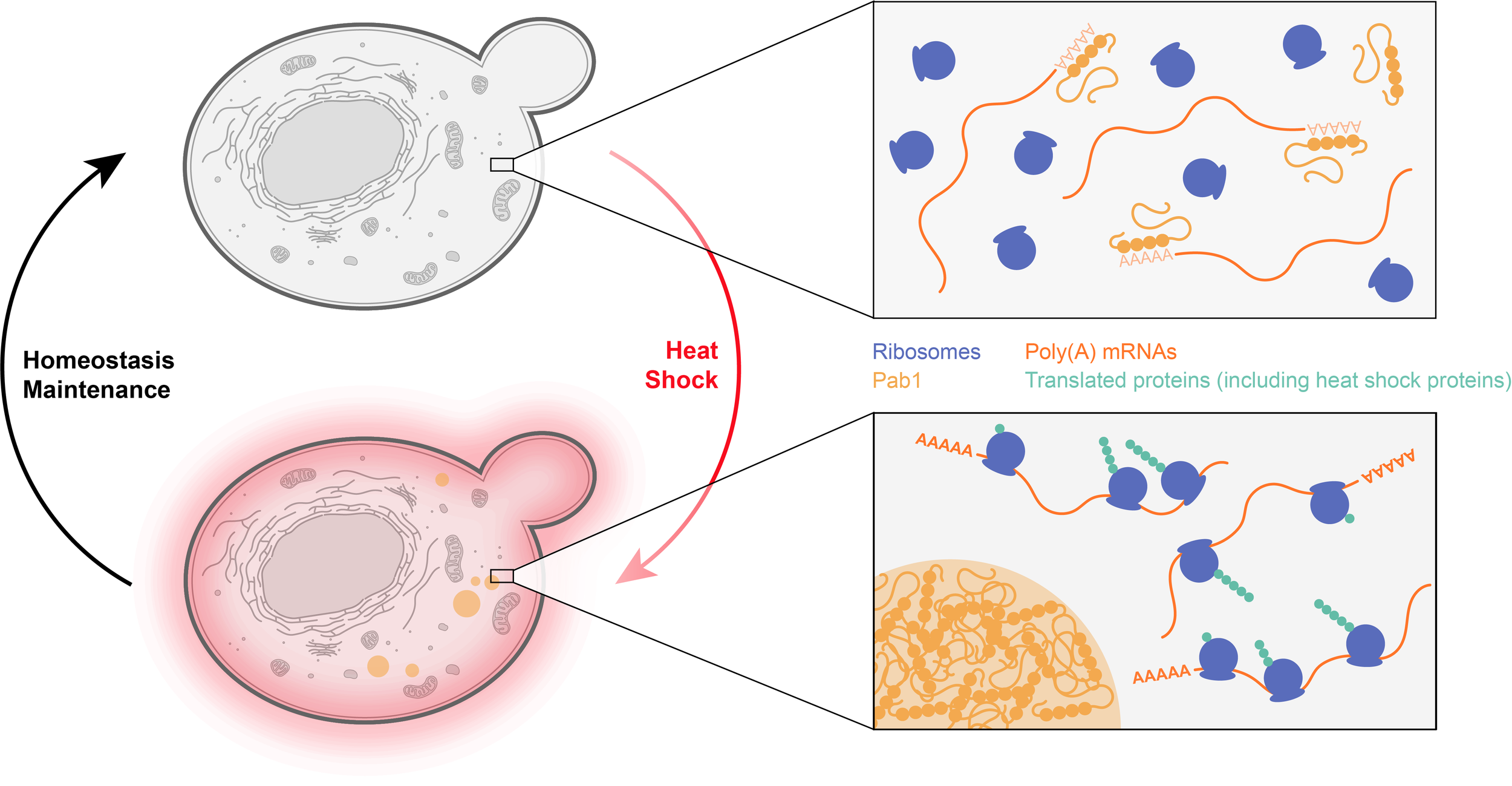
One important advantage of phase separated structures is that all of their potential functions can be switched on and off extremely rapidly by controlling their formation and dissolution. At the solubility limit, even tiny changes in a parameter (such as concentration or temperature) can sharply induce phase transitions. The reversible assembly of a biocondensate could thus serve as a sensor to various stresses and induce appropriate homeostatic responses.
Example: The budding yeast protein Pab1 binds to the poly(A) 5’ sequence of mRNAs, preventing their recognition by the translation machinery. Upon heat shock, Pab1 undergoes rapid phase separation, releasing bound mRNAs. This suggests that translation of mRNAs with A-rich 5ʹ untranslated regions, which includes many mRNAs coding for proteins involved in the heat shock response, will be selectively enhanced under such conditions.
3. Pathological biocondensates
The sensitivity of phase-separating proteins to changing physico-chemical conditions also comes with a cost. An increasing number of recent studies link abnormal condensed phases to a number of diseases.
Phase separation can lead to assemblies of various material states and properties. Recent in vitro studies showed that reconstituted liquid droplets can convert into a more solid-like state with time. They first harden into gels, before forming crystal-like, fibrillar aggregates, which are often much more stable. This maturation process has been called molecular aging and it seems to affect many types of liquid droplets in vitro.
Evidence for such molecular aging processes in cells remains scarce so far, suggesting that cells regulate the properties of their biocondensates to keep them in a dynamic, liquid-like state. The driving force for these additional transitions seems to be at least partially sequence-encoded. Mutations in phase separating proteins have been linked to abnormal biocondensates and the development of various diseases, including cancer and neurodegeneration.
References
Alberti S. Phase separation in biology. Curr Biol. 2017;27(20):R1097-R1102. doi:10.1016/j.cub.2017.08.069
Alberti S, Gladfelter A, Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell. 2019;176(3):419-434. doi:10.1016/j.cell.2018.12.035
Alberti S, Hyman AA. Are aberrant phase transitions a driver of cellular aging?. Bioessays. 2016;38(10):959-968. doi:10.1002/bies.201600042
Alberti S, Hyman AA. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol. 2021;22(3):196-213. doi:10.1038/s41580-020-00326-6
Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18(5):285-298. doi:10.1038/nrm.2017.7
Borcherds W, Bremer A, Borgia MB, Mittag T. How do intrinsically disordered protein regions encode a driving force for liquid-liquid phase separation?. Curr Opin Struct Biol. 2021;67:41-50. doi:10.1016/j.sbi.2020.09.004
Brangwynne, C. P., Eckmann, C. R., Courson, D. S., Rybarska, A., Hoege, C., Gharakhani, J., Juelicher, F., & Hyman, A. A. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science, 324(5935), 1729–1732.
Brini E, Fennell CJ, Fernandez-Serra M, Hribar-Lee B, Lukšič M, Dill KA. How Water's Properties Are Encoded in Its Molecular Structure and Energies. Chem Rev. 2017;117(19):12385-12414. doi:10.1021/acs.chemrev.7b00259
Choi, J.-M., Holehouse, A. S., & Pappu, R. V. (2020). Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annual Review of Biophysics, 49, 107–133.
Ditlev JA, Case LB, Rosen MK. Who's In and Who's Out-Compositional Control of Biomolecular Condensates. J Mol Biol. 2018;430(23):4666-4684. doi:10.1016/j.jmb.2018.08.003
Ganser LR, Myong S. Methods to Study Phase-Separated Condensates and the Underlying Molecular Interactions. Trends Biochem Sci. 2020;45(11):1004-1005. doi:10.1016/j.tibs.2020.05.011
He S, Chou HT, Matthies D, et al. The structural basis of Rubisco phase separation in the pyrenoid. Nat Plants. 2020;6(12):1480-1490. doi:10.1038/s41477-020-00811-y
Hirose T, Virnicchi G, Tanigawa A, et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol Biol Cell. 2014;25(1):169-183. doi:10.1091/mbc.E13-09-0558
Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39-58. doi:10.1146/annurev-cellbio-100913-013325
Klosin A, Oltsch F, Harmon T, et al. Phase separation provides a mechanism to reduce noise in cells. Science. 2020;367(6476):464-468. doi:10.1126/science.aav6691
Larson AG, Elnatan D, Keenen MM, et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. 2017;547(7662):236-240. doi:10.1038/nature22822
Li, P., Banjade, S., Cheng, H.-C., Kim, S., Chen, B., Guo, L., Llaguno, M., Hollingsworth, J. V., King, D. S., Banani, S. F., Russo, P. S., Jiang, Q.-X., Nixon, B. T., & Rosen, M. K. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature, 483(7389), 336–340.
Lyon AS, Peeples WB, Rosen MK. A framework for understanding the functions of biomolecular condensates across scales. Nat Rev Mol Cell Biol. 2021;22(3):215-235. doi:10.1038/s41580-020-00303-z
Martin, E. W., Holehouse, A. S., Peran, I., Farag, M., Incicco, J. J., Bremer, A., Grace, C. R., Soranno, A., Pappu, R. V., & Mittag, T. (2020). Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science, 367(6478), 694–699.
Martin EW, Mittag T. Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry. 2018;57(17):2478-2487. doi:10.1021/acs.biochem.8b00008
Mitrea DM, Chandra B, Ferrolino MC, et al. Methods for Physical Characterization of Phase-Separated Bodies and Membrane-less Organelles. J Mol Biol. 2018;430(23):4773-4805. doi:10.1016/j.jmb.2018.07.006
Riback JA, Katanski CD, Kear-Scott JL, et al. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell. 2017;168(6):1028-1040.e19. doi:10.1016/j.cell.2017.02.027
Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017; 357(6357):eaaf4382. doi:10.1126/science.aaf4382
Spannl S, Tereshchenko M, Mastromarco GJ, Ihn SJ, Lee HO. Biomolecular condensates in neurodegeneration and cancer. Traffic. 2019;20(12):890-911. doi:10.1111/tra.12704
Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH. Phase separation drives heterochromatin domain formation. Nature. 2017;547(7662):241-245. doi:10.1038/nature22989
Su X, Ditlev JA, Hui E, et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 2016;352(6285):595-599. doi:10.1126/science.aad9964
Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell. 2012;149(6):1188-1191. doi:10.1016/j.cell.2012.05.022
Terms of Use: Creative Commons Licensing CC BY 4.0 - https://creativecommons.org/licenses/by/4.0/